Introduction to Electron Configuration
Electron configuration is a fundamental concept in atomic structure and quantum mechanics. It describes how electrons are arranged within an atom’s energy levels and subshells. Understanding electron configuration is crucial for comprehending the behavior of atoms, chemical bonding, and the periodic table.
Electron configuration is based on the principles of orbital theory and quantum numbers. According to the Pauli exclusion principle, each atomic orbital can accommodate a maximum of two electrons with opposite spins. The arrangement of electrons in atomic orbitals follows specific rules, including the Aufbau principle and Hund’s rule.
To represent electron configuration, we use a notation that includes the energy level, subshell, and the number of electrons in each subshell. The energy levels are represented by numbers (1, 2, 3, etc.), while the subshells are denoted by letters (s, p, d, f). The number of electrons in each subshell is indicated by superscripts.
For example, let’s consider the electron configuration of the element carbon (C), which has an atomic number of 6. The electron configuration of carbon can be written as 1s^2 2s^2 2p^2. This means that carbon has two electrons in the 1s subshell, two electrons in the 2s subshell, and two electrons in the 2p subshell.
The electron configuration of an atom determines its chemical properties and reactivity. The outermost energy level, known as the valence shell, contains the valence electrons, which are involved in chemical bonding. The arrangement of electrons in the valence shell determines how atoms interact with each other to form compounds.
By understanding electron configuration, scientists can predict the behavior of elements and their compounds. The periodic table is organized based on electron configuration, allowing us to identify patterns in the properties of elements.
In summary, electron configuration provides a systematic way to describe the arrangement of electrons in an atom’s orbitals. It plays a crucial role in understanding atomic structure, chemical bonding, and the behavior of elements. By using quantum mechanics and the principles of orbital theory, we can determine the electron configuration of any element and gain insights into its properties.
Understanding the Basics of Electron Configuration
Electron configuration is a fundamental concept in atomic structure and quantum mechanics. It describes the arrangement of electrons within an atom’s energy levels and subshells. By understanding electron configuration, we can gain insights into an element’s properties, its behavior in chemical reactions, and its position in the periodic table.
Definition of Electron Configuration
Electron configuration refers to the distribution of electrons in atomic orbitals. Atomic orbitals are regions of space where electrons are most likely to be found. The arrangement of electrons follows specific rules governed by quantum numbers, such as the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms).
To write the electron configuration of an element, we use a specific notation that indicates the energy levels, subshells, and number of electrons in each subshell. The order in which electrons fill the orbitals is determined by the Aufbau principle, Hund’s rule, and the Pauli exclusion principle.
The electron configuration is written in a shorthand notation using the periodic table as a guide. The main energy levels are represented by numbers (1, 2, 3, etc.), while the subshells are represented by letters (s, p, d, f). The superscript numbers indicate the number of electrons in each subshell.
For example, the electron configuration of carbon (atomic number 6) is 1s² 2s² 2p². This means that carbon has two electrons in the 1s orbital, two electrons in the 2s orbital, and two electrons in the 2p orbital.
Importance of Electron Configuration
Electron configuration plays a crucial role in understanding the behavior of atoms and their interactions with other atoms. Here are some key reasons why electron configuration is important:
Chemical Bonding: The electron configuration of an atom determines its ability to form chemical bonds. Elements with similar electron configurations tend to exhibit similar chemical properties and can form bonds in predictable ways. For example, elements in the same group of the periodic table have the same number of valence electrons, which influences their reactivity.
Energy Levels and Stability: The arrangement of electrons in energy levels and subshells affects an atom’s stability. Atoms tend to be more stable when their energy levels and subshells are fully or half-filled. This stability is a result of the Pauli exclusion principle, which states that no two electrons in an atom can have the same set of quantum numbers.
Periodic Trends: Electron configuration helps explain periodic trends in the periodic table. For example, as we move across a period from left to right, the electron configuration changes, leading to variations in atomic size, ionization energy, and electron affinity.
Quantum Chemistry: Electron configuration is a fundamental concept in quantum chemistry. It provides the basis for understanding the electronic structure of molecules, bonding patterns, and the behavior of electrons in chemical reactions.
In summary, electron configuration is a vital concept in understanding the structure and behavior of atoms. It allows us to predict an element’s properties, its reactivity, and its position in the periodic table. By studying electron configuration, we can delve deeper into the fascinating world of atomic theory and quantum chemistry.
The Principles Guiding Electron Configuration
The Aufbau Principle
The Aufbau Principle is a fundamental principle in atomic structure and electron arrangement. It states that electrons fill atomic orbitals in a specific order, starting from the lowest energy level and gradually filling higher energy levels. This principle is based on the concept of quantum numbers and the Pauli Exclusion Principle.
To understand the Aufbau Principle, we need to delve into the concept of atomic orbitals. Atomic orbitals are regions of space where electrons are most likely to be found. They are characterized by quantum numbers, which describe the energy, shape, and orientation of the orbitals.
In the Aufbau Principle, electrons are filled into orbitals according to their energy levels. The lowest energy level, known as the first shell, is filled before moving on to the higher energy levels. Within each energy level, the orbitals are filled in a specific order based on their energy.
The order of filling orbitals follows a pattern known as the “orbital filling diagram.” This diagram shows the order in which the orbitals are filled, starting with the 1s orbital, followed by the 2s, 2p, 3s, and so on. Each orbital can hold a maximum of two electrons, with opposite spins according to the Pauli Exclusion Principle.
Hund’s Rule
Hund’s Rule is another principle that guides electron configuration. It states that when filling orbitals of the same energy level, electrons will occupy separate orbitals with parallel spins before pairing up. This rule ensures that electrons are distributed in a way that maximizes the stability of the atom.
To understand Hund’s Rule, let’s consider the example of the 2p orbitals. The 2p orbitals consist of three orbitals: 2px, 2py, and 2pz. According to Hund’s Rule, when filling these orbitals, electrons will first occupy each orbital with the same spin before pairing up.
For instance, if we have three electrons to fill in the 2p orbitals, they will occupy the 2px, 2py, and 2pz orbitals with the same spin before pairing up. This distribution of electrons with parallel spins maximizes the stability of the atom.
Pauli Exclusion Principle
The Pauli Exclusion Principle is a fundamental principle in quantum mechanics that states that no two electrons in an atom can have the same set of quantum numbers. This principle ensures that each electron in an atom is unique and occupies a specific orbital.
According to the Pauli Exclusion Principle, each orbital can hold a maximum of two electrons with opposite spins. This means that if an orbital is already occupied by one electron, the next electron must have an opposite spin to occupy the same orbital.
The Pauli Exclusion Principle plays a crucial role in determining the electron configuration of an element. By following the principles of the Aufbau Principle and Hund’s Rule, and considering the restrictions imposed by the Pauli Exclusion Principle, we can determine the electron configuration of any element.
In summary, the principles guiding electron configuration, including the Aufbau Principle, Hund’s Rule, and the Pauli Exclusion Principle, provide a framework for understanding how electrons are arranged in atoms. By following these principles and considering the energy levels, orbitals, and quantum numbers, we can determine the electron configuration of any element and gain insights into its chemical properties.
How to Determine Electron Configuration
Finding Electron Configuration: Step-by-step Guide
Determining the electron configuration of an atom is essential in understanding its atomic structure and chemical behavior. The electron configuration describes the arrangement of electrons within the atom’s energy levels and subshells. By following a step-by-step guide, you can easily determine the electron configuration of any element.
Start by identifying the atomic number of the element. The atomic number represents the number of protons in the nucleus of an atom and determines its position in the periodic table.
Use the Aufbau principle to determine the order in which electrons fill the atomic orbitals. According to this principle, electrons occupy the lowest energy orbitals first before moving to higher energy levels. The order of filling is as follows: 1s, 2s, 2p, 3s, 3p, 4s, and so on.
Apply the Pauli exclusion principle, which states that each orbital can hold a maximum of two electrons with opposite spins. This means that if an orbital is already occupied by one electron, the next electron must have an opposite spin.
Utilize the Hund’s rule to distribute electrons within a subshell. Hund’s rule states that when filling degenerate orbitals (orbitals with the same energy), electrons will occupy separate orbitals with parallel spins before pairing up.
Continue filling the orbitals and subshells in the order determined by the Aufbau principle until you have accounted for all the electrons in the atom.
Write the electron configuration using the following notation: the principal quantum number (n), the subshell (s, p, d, or f), and the superscript representing the number of electrons in that subshell. For example, the electron configuration of carbon (atomic number 6) would be written as 1s² 2s² 2p².
Electron Configuration and the Periodic Table
The electron configuration of an atom is closely related to its position in the periodic table. The periodic table is organized based on the increasing atomic number, which directly correlates with the electron configuration.
The periodic table is divided into periods (rows) and groups (columns). Each period represents a new energy level, while each group shares similar electron configurations in their outermost energy level, also known as the valence electrons.
Understanding the electron configuration allows us to predict an element’s chemical behavior and its ability to form chemical bonds. Elements with similar electron configurations often exhibit similar chemical properties and tend to form similar types of compounds.
Electron Configuration for Specific Elements
To determine the electron configuration for specific elements, you can refer to the periodic table and follow the steps mentioned earlier. Let’s take the example of oxygen (atomic number 8).
Oxygen has 8 electrons, so we start by filling the orbitals in the order of increasing energy: 1s² 2s² 2p⁴.
The electron configuration of oxygen can be represented as 1s² 2s² 2p⁴.
By following this process, you can determine the electron configuration for any element on the periodic table.
In conclusion, understanding electron configuration is crucial in comprehending the atomic structure and chemical behavior of elements. By following a step-by-step guide and utilizing the periodic table, you can easily determine the electron configuration for specific elements.
Electron Configuration and Chemical Properties
How Electron Configuration Affects Chemical Properties
The electron configuration of an atom refers to the arrangement of electrons within its atomic orbitals. It plays a crucial role in determining the chemical properties of an element. By understanding the electron arrangement, we can gain insights into how atoms interact and form chemical bonds.
The electron configuration is governed by various principles and rules, such as the Pauli exclusion principle, the Aufbau principle, and Hund’s rule. These principles help us determine the distribution of electrons in different energy levels, subshells, and orbitals.
The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. This means that each electron must have a unique combination of quantum numbers, including the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms).
To write the electron configuration of an element, we start by listing the energy levels (represented by the principal quantum number, n) in ascending order. Within each energy level, we list the subshells (represented by the azimuthal quantum number, l) in order of increasing energy. The subshells are labeled as s, p, d, and f.
Next, we determine the number of electrons in each subshell. The maximum number of electrons that can occupy a subshell is given by 2(2l + 1). For example, the s subshell can hold a maximum of 2 electrons, the p subshell can hold a maximum of 6 electrons, the d subshell can hold a maximum of 10 electrons, and the f subshell can hold a maximum of 14 electrons.
Following the Aufbau principle, we fill the subshells in order of increasing energy. This means that the 1s subshell is filled before the 2s subshell, and so on. Within a subshell, we use Hund’s rule, which states that electrons will occupy separate orbitals within the same subshell before pairing up. This maximizes the total spin of the electrons, resulting in greater stability.
The electron configuration of an element provides valuable information about its chemical behavior. Elements with similar electron configurations often exhibit similar chemical properties. This is why elements are organized in a systematic manner in the periodic table, based on their electron configurations.
Electron Configuration and Valence Electrons
Valence electrons are the electrons in the outermost energy level of an atom. They are responsible for the majority of an element’s chemical properties, as they participate in chemical bonding and reactions. The electron configuration of an atom directly determines the number of valence electrons it possesses.
To determine the number of valence electrons in an atom, we look at the electron configuration and identify the outermost energy level. The electrons in this energy level are the valence electrons. For example, in the electron configuration of oxygen (1s² 2s² 2p⁴), the outermost energy level is the second energy level (2s² 2p⁴), and it contains 6 valence electrons.
The number of valence electrons influences an atom’s ability to form chemical bonds. Elements with a full outermost energy level (8 valence electrons for most elements) tend to be stable and unreactive. These elements are known as noble gases. On the other hand, elements with fewer than 8 valence electrons tend to be reactive and readily form chemical bonds to achieve a stable electron configuration.
Chemical bonding occurs when atoms share, gain, or lose electrons to achieve a more stable electron configuration. Atoms with similar valence electron configurations often exhibit similar bonding patterns. For example, elements in the same group of the periodic table have the same number of valence electrons and tend to form similar types of chemical bonds.
In summary, the electron configuration of an atom plays a crucial role in determining its chemical properties. By understanding the arrangement of electrons within atomic orbitals, we can gain insights into an element’s reactivity, bonding behavior, and overall chemical behavior.
Special Cases in Electron Configuration
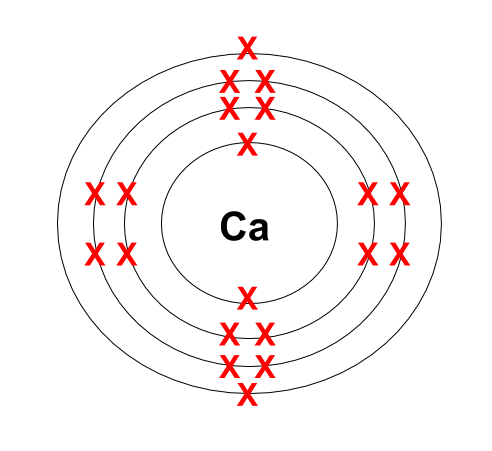
In the study of atomic structure and electron arrangement, there are certain special cases that arise when determining the electron configuration of elements. These cases involve anomalies in the expected order of filling electron orbitals and the use of noble gas configurations as a shorthand notation. Let’s explore these special cases in more detail.
Electron Configuration of Noble Gases
Noble gases, such as helium (He), neon (Ne), and argon (Ar), have completely filled electron shells. This stable configuration makes them unreactive and chemically inert. When writing the electron configuration of elements, we can use noble gas configurations as a starting point. For example, instead of writing the full electron configuration for potassium (K) as 1s2 2s2 2p6 3s2 3p6 4s1, we can use the noble gas configuration of argon ([Ar]) and simply add the remaining electrons: [Ar] 4s1. This shorthand notation saves time and space when representing electron configurations.
Anomalies in Electron Configuration: Chromium and Copper
The electron configuration of chromium (Cr) and copper (Cu) deviates from the expected order based on the Aufbau principle. According to the Aufbau principle, electrons fill orbitals in order of increasing energy. However, in the case of chromium and copper, one electron from the 4s orbital is promoted to the 3d orbital to achieve a more stable configuration. The electron configuration of chromium is [Ar] 3d5 4s1, and the electron configuration of copper is [Ar] 3d10 4s1. This anomaly is due to the exchange of energy levels between the 3d and 4s orbitals, resulting in a more favorable arrangement.
Electron Configuration for Excited States
In addition to the ground state electron configuration, atoms can also exist in excited states where electrons occupy higher energy levels. These excited states occur when electrons absorb energy and transition to higher orbitals. The electron configuration for excited states is denoted by adding an asterisk () after the noble gas configuration and specifying the additional electrons. For example, the excited state electron configuration of oxygen (O) can be written as [He] 2s2 2p4. This notation indicates that two additional electrons occupy the 2p orbital in the excited state.
Understanding these special cases in electron configuration is crucial for comprehending the principles of atomic theory, quantum mechanics, and chemical bonding. By considering the order of filling orbitals, the Pauli exclusion principle, Hund’s rule, and the use of noble gas configurations, we can accurately describe the distribution of electrons within an atom and predict its chemical behavior.
Now that we have explored the electron configuration of noble gases, the anomalies in electron configuration for chromium and copper, and the electron configuration for excited states, we have gained a deeper understanding of the intricate nature of electron arrangement and its significance in the field of quantum chemistry.
Practice and Application of Electron Configuration
Electron Configuration Practice Worksheet
To understand the practice and application of electron configuration, it is important to first grasp the concept of atomic structure and electron arrangement. Electron configuration refers to the distribution of electrons in an atom’s energy levels and orbitals. It is determined by the principles of orbital theory, quantum numbers, and the Pauli exclusion principle.
In electron configuration, atomic orbitals are organized into electron shells and energy levels. The periodic table provides a systematic order for filling these orbitals with electrons based on the atomic number of an element. The subshell configuration, which includes the values of the quantum numbers l and ml, is used to write the electron configuration of an element.
Let’s take an example to understand this better. Consider the element oxygen (O) with an atomic number of 8. To write its electron configuration, we start by filling the orbitals in order of increasing energy. The first two electrons occupy the 1s orbital, followed by two electrons in the 2s orbital. The remaining four electrons are distributed in the 2p orbitals. The electron configuration of oxygen can be written as 1s² 2s² 2p⁴.
The electron spin is represented by the quantum number ms, which can have values of +1/2 or -1/2. Hund’s rule states that when filling degenerate orbitals, electrons will occupy separate orbitals with parallel spins before pairing up. This rule helps in determining the electron distribution within a subshell.
Real-life Applications of Electron Configuration
The understanding of electron configuration has significant real-life applications, particularly in the field of chemistry. It plays a crucial role in explaining chemical bonding and the behavior of elements in various chemical reactions.
By knowing the electron configuration of an atom, we can determine the number and arrangement of valence electrons. Valence electrons are the outermost electrons involved in chemical bonding. The electron configuration provides insights into an element’s reactivity and its ability to form bonds with other elements.
Additionally, electron configuration helps in predicting an element’s position in the periodic table. Elements with similar electron configurations often exhibit similar chemical properties. This knowledge is essential for understanding periodic trends and the periodicity of elements.
In quantum chemistry, electron configuration is used to describe the arrangement of electrons in molecular orbitals. This information is crucial in understanding the electronic structure and properties of molecules.
In conclusion, electron configuration is a fundamental concept in atomic theory and quantum mechanics. It allows us to understand the distribution of electrons within an atom’s orbitals and energy levels. This knowledge has practical applications in chemistry, helping us explain chemical bonding, periodic trends, and the behavior of elements in various reactions.
Conclusion: The Significance of Electron Configuration in Chemistry
The electron configuration of an atom plays a crucial role in understanding its chemical behavior and properties. By examining the arrangement of electrons within an atom’s energy levels and orbitals, scientists can gain valuable insights into various aspects of atomic structure and chemical bonding.
One of the fundamental concepts in atomic structure is the use of quantum numbers to describe the distribution of electrons. These quantum numbers, such as the principal quantum number (n), azimuthal quantum number (l), magnetic quantum number (ml), and spin quantum number (ms), provide a framework for understanding the organization of electrons within an atom.
The electron configuration of an atom is determined by following certain rules and principles. The Aufbau principle states that electrons fill the lowest energy levels and orbitals first before moving to higher energy levels. This principle helps in determining the order in which electrons occupy different orbitals.
The Pauli exclusion principle states that no two electrons in an atom can have the same set of quantum numbers. This principle ensures that electrons within the same orbital have opposite spins, which helps in stabilizing the atom.
The Hund’s rule states that electrons occupy orbitals of the same energy level singly before pairing up. This rule helps in achieving maximum stability within the atom.
By understanding the electron configuration of an element, we can determine the number of electrons in each energy level, the number of valence electrons, and the overall stability of the atom. This information is crucial in predicting an element’s reactivity and its ability to form chemical bonds.
The periodic table provides a systematic arrangement of elements based on their electron configurations. It allows us to identify trends in properties and behavior as we move across a period or down a group. The electron configuration provides a basis for explaining these trends and understanding the periodicity of elements.
In summary, electron configuration is a fundamental concept in chemistry that helps us understand the behavior and properties of atoms. It provides insights into the organization of electrons within an atom, the stability of elements, and their ability to form chemical bonds. By studying electron configuration, scientists can unlock the secrets of atomic structure and delve deeper into the fascinating world of quantum chemistry.
Frequently Asked Questions
1. What is an electron configuration and why is it important?
Electron configuration refers to the distribution of electrons in an atom’s atomic orbitals. It’s crucial in understanding the chemical behavior of an atom. This is because the arrangement of electrons influences an atom’s ability to form bonds with other atoms.
2. How does the Aufbau Principle apply to electron configuration?
The Aufbau Principle, a key concept in quantum mechanics, states that electrons fill atomic orbitals starting at the lowest available energy levels before filling higher levels. In other words, electrons fill up the “lowest energy” orbitals first.
3. What does the electron configuration of an element on the periodic table represent?
The electron configuration of an element on the periodic table represents the distribution of electrons in atomic orbitals. Each element on the table has a unique electron configuration that corresponds to its atomic number.
4. Can the electron configuration of an atom change?
Yes, the electron configuration of an atom can change when it gains or loses energy. This can occur during chemical reactions, or when an atom absorbs or emits light. The electron can move from one energy level to another, altering the atom’s electron configuration.
5. Why are some electron configurations of elements like Chromium and Copper unique?
Certain elements like Chromium and Copper have unique electron configurations because they can achieve greater stability by having filled or half-filled subshells. For instance, Chromium’s electron configuration is [Ar] 3d5 4s1, not [Ar] 3d4 4s2 as one might expect.
6. What is the role of quantum numbers in electron configuration?
Quantum numbers describe the properties of atomic orbitals and the electrons in those orbitals. They are crucial in determining the electron configuration of an atom. There are four quantum numbers: the principal quantum number, the azimuthal quantum number, the magnetic quantum number, and the spin quantum number.
7. What is the relationship between electron configuration and valence electrons?
The electron configuration of an atom can reveal how many valence electrons (electrons in an atom’s outermost shell) it has. Valence electrons are important because they are involved in forming chemical bonds with other atoms.
8. How does the Pauli Exclusion Principle relate to electron configuration?
The Pauli Exclusion Principle states that no two electrons in an atom can have the same four quantum numbers. This principle influences electron configuration, as it prevents more than two electrons from occupying the same atomic orbital.
9. How does Hund’s Rule affect electron configuration?
Hund’s Rule states that electrons prefer to occupy separate orbitals in a subshell with parallel spins before doubling up within orbitals. This rule influences the electron configuration of an atom, particularly when dealing with atoms that have partially filled subshells.
10. How does the electron configuration contribute to chemical bonding?
Electron configuration is crucial in chemical bonding because it determines how an atom can bond with others. Atoms tend to bond in a way that allows them to achieve a stable electron configuration. For example, atoms often gain, lose or share electrons to attain a full outer electron shell, leading to the formation of ionic or covalent bonds.
Also Read:
- Ruthenium electron configuration
- Europium electron configuration
- Einsteinium electron configuration
- Xenon electron configuration
- Molybdenum electron configuration
- Indium electron configuration
- Promethium electron configuration
- Selenium electron configuration
- Ni2 electron configuration
- Phosphorus electron configuration

The TechieScience Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the TechieScience.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.
