Any cell is said to be a mass of the cytoplasm that is guarded well by a cell membrane. They are all microscopic in size.
A cell in a hypotonic solution, has a net movement for water from the part of the solution in the body. A cell that is placed into a hypotonic solution shall seem to swell and then expand till it shall burst via a process called cytolysis.
A solution that is hypotonic or a cell in a hypotonic solution has a concentration that is lower than that of the solutes of the other solution. In the term of biology, the solution that is out of cell is said to be hypotonic only if it has less concentration for solutes considering to that of cytosol. There is a diffusion of water in the cell, for osmotic pressure and then the cell leads to often being turgid or also bloated.
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. Some examples of solutions are salt water, rubbing alcohol, and sugar dissolved in water. When you look closely, upon mixing salt with water, you can’t see the salt particles anymore, making this a homogeneous mixture

Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affecting imbibition. A hypotonic solution has a lower concentration of solutes than another solution.
Solution are said to be a mixture of a compound made of a solvent and a solute. The substance that is the one that is present in more concentration is called as solvent and the one that is in less level is said to be solute. A salt water is a good example of hypotonic solution. Thus, cell in a hypotonic solution shall have less solute concentration than the cell. An iso-osmolar solution can be hypotonic if the solute is able to penetrate the cell membrane.
What happens to a red blood cell in a hypotonic solution?
The very simple definition of tension or more tone means to have a large osmotic pressure that the rest medium or fluid.
The time when cell in a hypotonic solution shall have lower concentration, the red blood cell kept in this solution shall have a free water net movement in the cell. This means less water than the cell.
On having a cell in a hypotonic solution or rather a red blood cell in it shall have it bloated up and might explode. If it is kept in a hypertonic mode, it shall shrivel that shall make the cytoplasm dense and the components shall remain concentrated and also can die. Thus, for this reason a plant cell seems to be ideal. To prevent the process of burst, the cell needs isotonic mode.

After having the red blood cell in a hypotonic solution there shall be seen a net movement for free water inside the cell. This phase shall outcome in an increase volume in the cell with having less concertation of solute. The solution shall end up with finally high level of concentration. It would eventually lead to it swelling up and then bursting in the method called hemolysis.
To avoid situation like this, cell in a hypotonic solution can never be the red blood cell. Thus to level this up or avoid the process of hemolysis, on needs to place the cell in a solution called to be isotonic solution. This shall have 0.9% m/v of NaCl and glucose shall be in a concentration of 5% m/v. A solution is isotonic when there is equal amount if solutes on both side of membrane and no swelling or shrinking is seen.
Any example of isotonic solution can be the normal saline water that is 0.9% in concentration and the lactated ringers. These fluids are said to be useful at the time when there is a huge loss of body fluids from any trauma, dehydration, vomiting, diarrhea, blood loss or nausea. This term is used both in biology and in chemistry for transport across the semipermeable membrane.
What happens to an animal cell in a hypotonic solution?
Any cell in a hypotonic solution shall, have less amount if solute concentration and more solvent in it. Osmosis is the process involved in it.
The solutions that are hypotonic shall have less water than the cell. Thus with animal cell in a hypotonic solution shall be filled up with water completely and then shall burst. Pure water and tap water are hypotonic.
An animal cell is a eukaryotic cell type that do not have a cell all yet seems to have a nucleus that has a membrane and is true. It also has several other cellular organelles. The contents that it has are cytoskeleton, centrosome, lysosome, mitochondria, Golgi apparatus. Any typical cell that is of an animal shall have cytosol, a cell membrane, the organelles and cytoplasmic structure.
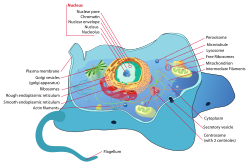
The process of osmosis takes place at the time when animal cell in a hypotonic solution. Thus for this, the animal and plant cell seem to both appear a lot plump while placed in a hypotonic solution. After this has been seen under the microscope, the vacuoles seem to appear a lot larger considering only the plant cell. It has more of solute and net movement. It is all a result of the method called as osmosis.
The swelling of a cell in a hypotonic solution is for the less amount of solute in it and the net movement of water in the cell causes a breaking or swelling of the cell. There is a movement of water at a place where there is low outside fluid or osmolality to an area that has more of it. The cell shall also want to expand. Just like the animal cell, the plant cell shall mot burst. The increase in solute level leads to it being broken finally.
What happens to a plant cell when placed in a hypotonic solution?
Both the plants and animal ell are said to be different and thus perform and show different types of property when placed to conditions.
When a plant cell in a hypotonic solution is exposed it shows up osmosis that takes up water and then begins to swell up. There is a rigid wall in the plants that help prevent the cell from getting it busted making it turgid.
Osmosis is said to be a process having the spontaneous path or water diffusion system in rest of the solvents via a semipermeable membrane. It is the simple movement concerned to water from the place of high concentration for water to the place of low level water. It helps in getting the two of the solutions separated by having the solute concentrated.

If there is a link made between the two areas via a membrane, then the one that has more of the solute that the rest then the eater shall flow from the second point to the first. The ability of the solution to have the water move is said to be tonicity. The tonicity of any of the solution shall be called as osmolality. Concentration and solubility is directly related to each other.
Water seems to enter the cell for the plant and the also the plasma membrane that tends to swell up and the push up against the walls of cell. If the plant cell in a hypotonic solution has a concentration of solute less than the within if the cell the water shall tend to enter the cell via osmosis. The cell shall swell but with zero lyse as the cell wall gives the structure to expand. Thus, the cell membrane shall press up the wall and make a turgor force that shall give the support to plant.
The cell after attaining the osmolality in the hypotonic solution, shall not burst but expand. But the animal cell in a hypotonic solution shall explode. This is so as the plant cell have a wall for the cell that is rigid all the way via the plasma membrane. On getting to swell with the water they shall turn turgid. Hypotonic solutions also help in making the vegetables like bell pepper turn crisp.
What happens to a bacterial cell in a hypotonic solution?
Usually if there is a placement of cell in a hypotonic solution it is quite likely to get ruptured or swell up due to its tonicity.
If a bacterium cell is kept in a hypotonic solution, it shall seem to rupture by having the cell swell due to the gradient of osmotic pressure within the cell of the bacteria.
The bacteria are microorganism that have a cell boundary and is simple that that of the rest of the organism, they have their own center of control that have genetic data and is kept in a single loop for the DNA. Some of the bacteria do have an extra genetic circle of cell material and is called to be plasmid rather than being a nucleus. They are the prokaryotic cell type.

For the instance of osmotic pressure and the gradient that is made the relative presence of the hypertonic solution in the cell makes it swell and in the method it becomes much slow and some of them are finally resistance to each other in action by all means of the cell wall. If there is the control of water, the cell might lead to damage and burst. The plasma membrane helps in getting the pressure kept normal with bacteria having straight characters.
Most of the cell wall of the bacteria, fungi and algae have a cell wall that is rigid and are able to tolerate the osmolality and then enjoy the surrounding being hypotonic. If the solution seems to be hypertonic then the water that is in, then cell shall be able to leave it and then the bacteria will be shrinking. The cell movement outside the water is called to be osmosis. There is a prevention of the cell from expansion and thus have lysis.
Can a Hyperosmotic solution be hypotonic?
Osmosis is said to be the solute number that gets dissolve while tonicity leads to having no units. Osmolality is said to be comparison of two solutions.
Yes, it can be so. If the cell is placed in a hyperosmotic solution but the hypotonic part is like dextran of 10% concentration, there shall be a water movement. Thus, a hyperosmotic solution can be hypotonic.
With also have this question of can hyperosmotic solution can be hypotonic, it is not always needed to be so. Yet, the hyperosmotic solutions shall always remain hypotonic. There is a good response for the widespread presentation of tonicity and osmolality that had a good result. It also needs bit of an additional arrangement.

Hyperosmotic stress in most mammalian cells causes cell shrinkage due to osmotic efflux of water leading to increases in intracellular ionic strength. Hyperosmotic solution has been shown to disrupt mating pairs at two different time points. First, as described earlier, osmotic solution will erase the preparative changes brought about by “initiation”. The increase of intracellular ions and the accompanying influx of water cause RVI.
A solution can be either isotonic or hyperosmotic and both. If a cell needs to be placed in a hypertonic solution, there will be a net flow of water out the cell and then the cell shall loose volume. A solution shall be hypertonic in a cell and thus its solute shall be having a high concentration. There is always a swelling due to influx and the cell shrinks.
Can a Hyperosmotic solution be isotonic?
A solution can be either be isotonic or also be hyperosmotic. Hyperosmotic refers to the capacity of achieving more than the normal osmosis.
The solutes that are hard to penetrate or actually have zero penetration via the cell membrane, thus the water movement across the membrane of the cell shall be able to take place to reach stability. Solutions of equal solute concentration are isotonic.
Tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially permeable cell membrane. Isotonic is a term used to describe solutions and chemistry and, sometimes, muscles in human biology. In chemistry, a solution is said to be isotonic when it has the same concentration of solutes as another solution across a semipermeable membrane. The use of isotonic in human anatomy is used more rarely.
The very term hyperosmotic means the property of having a pressure with less osmosis. This means that the molecules in solute shall number in a single side of the membrane that shall allow only specific molecules to pass via the low side than the one that has on the rest side considering the number of solute. The water molecules shall be travelling fast and via the cell membrane that shall have the particles to solute in.
Hyperosmotic vs hypertonic
The solutions that tend to have less number of solute is called to be hypotonic. The word hypo means less and is same to hyperosmotic.
The solutions that are hyperosmotic are never always hypertonic. But the hypsometric ones are always said to be hypotonic. This depends on the tonicity and the osmolality of the solutions.
In the solutions that are hyperosmotic the solutes tend to move from the place where the surrounding has a high osmotic pressure that the rest part of the solution. On the other part, the hypotonic have the solutes that travel from the rea that have high part of concertation and move to the less area concentration or the surrounding.
In simple way, hyperosmotic refers to the character that has a pressure high is so osmolality while hypotonic refers to the feature of having an osmotic pressure that is less. Hyperosmotic also have pressure that is high in the rest of the area concerned to the cell while a cell in a hypotonic solution shall have less pressure out. Solution in hyperosmotic tend to move from the solution to rest while opposite in hypotonic.

Hyperosmotic stress results from an extracellular osmolyte or solute concentration in the serum or medium that is higher than physiological, and high in comparison to the intracellular environment. Hyperosmolality is classified as hypertonic or isotonic according to whether cell shrinkage occurs. A hypertonic medium contains solutes that are relatively membrane impermeable, such as peptides, metabolites, and small ions.
Also Read:
- Photocell sensors
- Is cyanobacteria unicellular or multicellular
- Is fungi multicellular or unicellular
- Does fungi have a cell wall
- Bacterial cell type
- Are yeast multicellular
- Fuel cell aircraft
- Eukaryotic cells vs bacterial cells
I am Ankita Chattopadhyay from Kharagpur. I have completed my B. Tech in Biotechnology from Amity University Kolkata. I am a Subject Matter Expert in Biotechnology. I have been keen in writing articles and also interested in Literature with having my writing published in a Biotech website and a book respectively. Along with these, I am also a Hodophile, a Cinephile and a foodie.
